10 questions parents have about the COVID vaccine for kids under 5
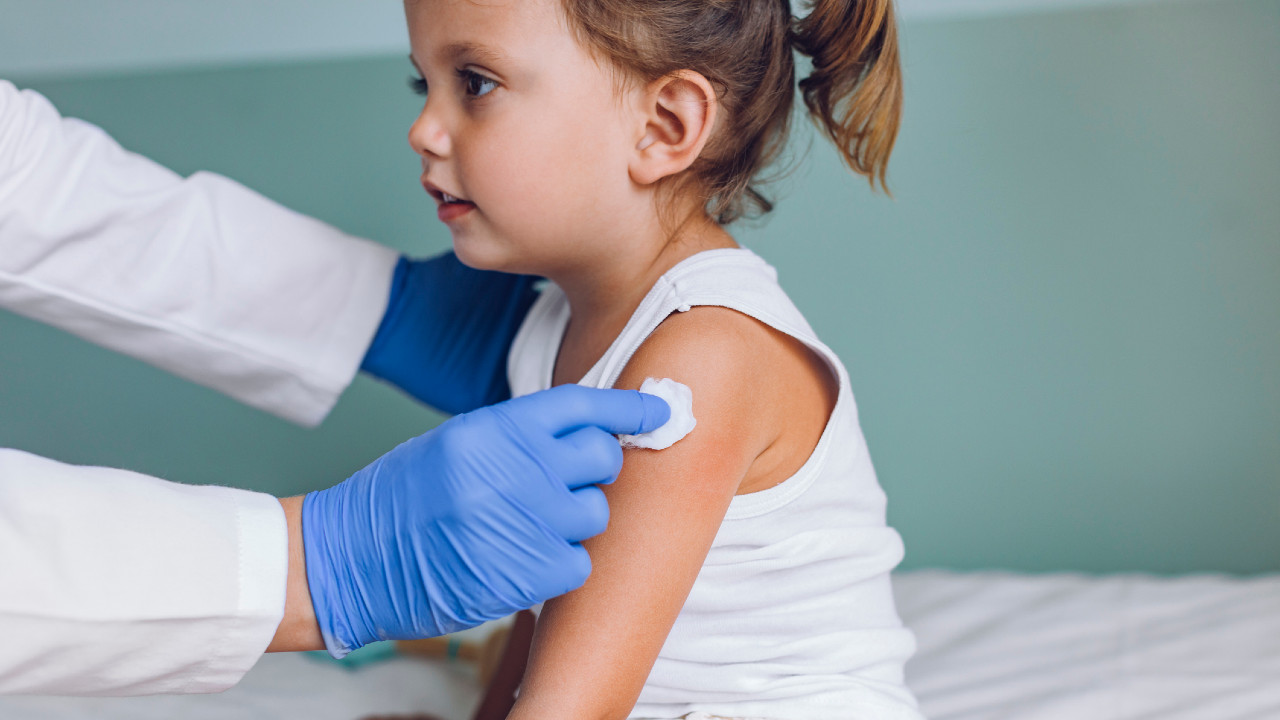
The first COVID-19 vaccine for children under the age of five has (finally!) been approved by Health Canada. Moderna?s vaccine, known as Spikevax, will now be available for babies, toddlers and preschoolers. With BA.4 and BA.5 variants on the rise, we answer parents’ most common questions about the under-5 vaccine.
1. What do we know about the recently approved Moderna COVID-19 vaccine"
Moderna?s vaccine uses messenger RNA (mRNA) technology, which teaches cells how to make a protein to trigger an immune response, and it?s a little-kid version of the vaccines already approved for older age groups.
The Moderna vaccine is a two-dose series for children aged six months to five years old. Each shot consists of 25 micrograms per shot, a quarter of the dosage approved for 18 plus. (The adult version of the vaccine is 100 micrograms and the version for six to 11-year-olds is 50 micrograms). While the doses in the clinical trials were given four weeks apart, the National Advisory Committee on Immunization (NACI), which provides recommendations for vaccine usage in Canada, advises waiting at least eight weeks between the first and second dose. (Kids who are immunocompromised may be eligible for three doses, spaced four to eight weeks apart.) That longer interval allows for kids to mount a better immune response, explains Jesse Papenburg, a paediatric infectious disease specialist at the McGill University Health Centre.
2. Why did the COVID-19 vaccine for the youngest kids...
| -------------------------------- |
|
|
Finding the Right School with John Catt Educational
31-10-2024 06:53 - (
moms )
Nine reasons to join Year 9 at Millfield
30-10-2024 06:58 - (
moms )













